Shelf Life Extension of Coronavac vaccine from 18 months to 24 months

Product Shelf Life Extension
The Coronavac vaccine’s expiration date has been extended from 18 months to 24 months based on the approval by the National Pharmaceutical Regulatory Agency (NPRA) on 23 June 2022.
The 24-month extension is given for the vaccine supplied by Pharmaniaga LifeScience Sdn Bhd with the registration number:
i) CoronaVac Suspension for Injection Covid-19 Vaccine (Vero Cell), Inactivated (MAL21036010ARZ)
ii) CoronaVac Suspension for Injection Covid-19 Vaccine (Vero Cell), Inactivated (MAL21046125ACSZ)
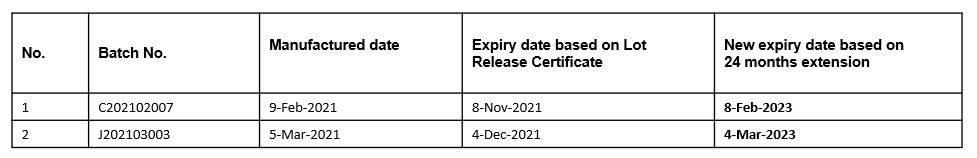
Product shelf life extensions have been granted based on latest stability data submitted by the manufacturer. The shelf life will be extended further once the data becomes available and approved by NPRA.
For the full listing, please visit NPRA’s website at www.npra.gov.my or click HERE.
